Application of a fluorescent dye-based microfluidic sensor for real-time detection of mAb aggregates
Abstract
The lack of process analytical technologies able to provide real-time information and process control over a biopharmaceutical process has long impaired the transition to continuous biomanufacturing. For the monoclonal antibody (mAb) production, aggregate formation is a major critical quality attribute (CQA) with several known process parameters (i.e., protein concentration and agitation) influencing this phenomenon. The development of a real-time tool to monitor aggregate formation is then crucial to gain control and achieve a continuous processing. Due to an inherent short operation time, miniaturized biosensors placed after each step can be a powerful solution. In this work, the development of a fluorescent dye-based microfluidic sensor for fast at-line PAT is described, using fluorescent dyes to examine possible mAb size differences. A zigzag microchannel, which provides 90% of mixing efficiency under 30 s, coupled to an UV–Vis detector, and using four FDs, was studied and validated. With different generated mAb aggregation samples, the FDs Bis-ANS and CCVJ were able to robustly detect from, at least, 2.5% to 10% of aggregation. The proposed FD-based micromixer is then ultimately implemented and validated in a lab-scale purification system, demonstrating the potential of a miniaturized biosensor to speed up CQAs measurement in a continuous process.
Abbreviations
-
- Bis-ANS
-
- 4-4-bis-1-phenylamino-8-naphthalene sulfonate
-
- CCVJ
-
- 9-(2-carboxy-2-cyanovinyl)julolidine
-
- DLS
-
- dynamic light scattering
-
- F/T
-
- freeze-thawing
-
- FD
-
- fluorescent dye
-
- FT
-
- flow through
-
- HIC
-
- hydrophobic interaction chromatography
-
- HMW
-
- high-molecular weight
-
- HT
-
- high-throughput
-
- LoD
-
- limit of detection
-
- PDMS
-
- poly(dimethylsiloxane)
-
- SEC
-
- size exclusion chromatography
-
- ThT
-
- thioflavin T
1 INTRODUCTION
The creation of process analytical technologies (PAT) for fast analytics and control is still one of the major challenges to tackle when implementing continuous bioprocessing into biopharmaceutical processes. To elicit a response for fluctuations in operational conditions, in-line or at-line sensors need to be placed within the manufacturing process to provide a real-time measurement of product critical quality attributes (CQAs).1 A common monitored CQA is protein aggregation, especially in the biomanufacturing of monoclonal antibodies (mAbs). Even though the presence of high molecular weight (HMW) species in the final formulation can enhance immune response, their appearance is inevitable.2 For example, the increasing protein concentration3 and agitation4 are known aggregation inducing factors. Hence, a PAT tool for the detection of mAb HMW species is crucial to allow to control their formation.
Fluorescent dyes (FD), such as 4-4-bis-1-phenylamino-8-naphthalene sulfonate (Bis-ANS), SYPRO Orange and Nile Red, are a widely used analytical technique to detect and study aggregation.5, 6 These dye's fluorescence intensifies (when compared to its intensity in the presence of the monomeric mAb form) in the presence of hydrophobic unfolded protein structures, a common characteristic in aggregate formation.5 In particular, the FD Bis-ANS binds to these hydrophobic residues through hydrophobic interactions of the aromatic rings, present in its structure.7 Additionally, a novel class of fluorescent molecular rotors, such as Thioflavin T (ThT), 9-(2-carboxy-2-cyanovinyl)julolidine (CCVJ) and Proteostat, have recently appeared as possible alternatives to the previously described classic probes. These molecular rotors rotate freely in solution and when their movement is restricted, these FDs emit fluorescence.8, 9 For example, when ThT binds to amyloid fibrils (insoluble proteinaceous materials formed during protein-misfolding events), through β-sheet-rich deposits, their FD's movement is constrained and it increases dramatically its fluorescence.10 Since both classes provide a fast, stable and straightforward result,11 these FDs can be taken into consideration when creating a real-time PAT tool to measure aggregation.12 To further speed up the analytical measurement, miniaturized sensors are considered a promising solution. The inherent short operation time, the minimal amounts of sample (nL or μL) and the easiness of fabrication and affordability are major benefits provided by the implementation of a miniaturized PAT tool.12 A zigzag microfluidic structure was designed and developed for a FD-based aggregate detection: this micromixer will then allow the mixing of a mAb sample with a FD.13 This zigzag microchannel, represented in Figure 1 (with its geometric measurements described in Table 1) provides a mixing efficiency of around 90% within 30 s. In total, this microchannel has 30 mixing zigzag units, adding to 26.25 mm of mixing length. Additionally, due to the low shear forces, this structure is expected not to alter the amount of aggregates during the measurement and, due to the low-pressure drop, the micromixer is easy to operate and fabricate. More information on the development of this structure and its characteristics can be found in São Pedro et al.13
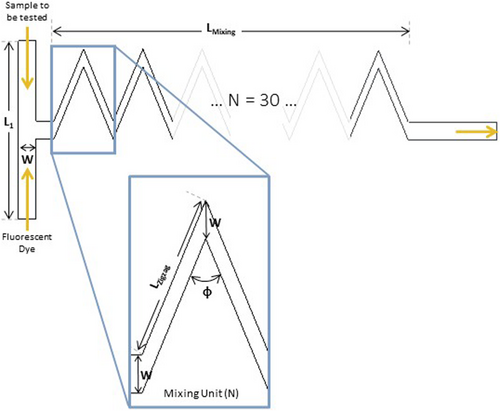
| Parameter | Measurement |
|---|---|
| L1 | 1000 μm |
| W | 100 μm |
| LZigzag | 440 μm |
| Φ | 45° |
| LMixing | 26.25 mm |
- Note: L1 corresponds to the transverse length of the T-junction, W to the width of the main channel, LZigzag to the length of the zigzag channel diagonal, Φ to the angle of the zigzag channel and LMixing to the mixing length, calculated based on the number of mixing units, 30, and the length of the zigzag channel diagonally.
Therefore, this microfluidic structure was designed to be able to produce an immediate aggregate detection in a continuous downstream process. However, this micromixer was only experimentally validated for its mixing efficiency. The ability of the proposed structure to actually detect aggregates still needs to be assessed. In this present work, first, a high-throughput (HT) screening of four different FDs is performed: the hydrophobic sensitive Bis-ANS and Nile Red and the molecular rotors ThT and CCVJ. The required FD concentration to be later employed in the micromixer is reached, as well as an early assessment into possible limits of detection intrinsic to each FD. After, resorting to different types of aggregates, the micromixer is used to identify aggregation using a UV–Vis detector: an increase in the UV signal is observed when aggregation is detected. Finally, the micromixer is validated in a chromatographic unit operation. Anion exchange (AEX) chromatography in an ÄKTA™ Avant unit was performed in flow-through (FT) mode to remove aggregates, with the micromixer implemented within the system. An increase in the UV signal was observed in the eluate, with the presence of aggregates later confirmed by analytical size exclusion chromatography (SEC-UPLC), while the FT (containing the product) did not produce any signal. A miniaturized FD-based PAT tool is then demonstrated, being able to robustly detect all types of aggregates which can arise in a downstream process.
2 MATERIALS AND METHODS
Poly(dimethylsiloxane) (PDMS) was purchased as a Sylgard 184 elastomer kit (Dow Corning, Midland, MI, USA) and dimethylsiloxane-(60%–70% ethylene oxide) block copolymer was acquired from Gelest (Pennsylvania, USA). Sodium phosphate monobasic dehydrate, ammonium sulfate and sodium citrate hydrate were purchased from Sigma-Aldrich (New Jersey, USA). Di-sodium hydrogen phosphate and sodium chloride (NaCl) were bought from VWR Chemicals (VWR International, Pennsylvania, USA). Acetic acid was obtained from Fluka (Honeywell, North Carolina, USA) and citric acid from J.T. Baker (VWR International, Pennsylvania, USA). The mAb employed in this study was supplied by Byondis B.V. (Nijmegen, The Netherlands), with an isoelectric point of 8.6.
Regarding the FDs used, ThT, CCVJ and Nile Red were purchased from Sigma-Aldrich (New Jersey, USA), whereas Bis-ANS was acquired from Invitrogen (Massachusetts, USA). For the preparation of the stock solutions, the dye ThT was dissolved in MilliQ water and filtered through a 0.2 μm Whatman syringe filter (Merck, New Jersey, USA). The exact concentration was calculated from measuring the UV absorbance at 412 nm, using a molar extinction coefficient of 36,000 M−1 cm−1.14 Bis-ANS was dissolved in methanol (Sigma-Aldrich, New Jersey, USA) and the exact concentration calculated at 385 nm, with a molar extinction of 16,790 M−1 cm−1.15 Finally, CCVJ and Nile Red were dissolved in dimethyl sulfoxide (Fluka, Massachusetts, USA), and the exact concentration calculated at 440 and 552 nm, with the molar extinction of 25,404 and 19,600 M−1 cm−1, respectively.8
2.1 Stressing of mAb formulations
The provided mAb was stored at a concentration of 6 mg mL−1 in sodium acetate buffer, pH 4.5, at −80°C. An early characterization of this sample was performed and the presence of 4% of aggregation was detected, being referred from here on as the storage aggregates. To remove these HMW species, size exclusion chromatography (SEC) was performed on an ÄKTA™ Avant system (Cytiva, Massachusetts, USA). An HiPrep™ 16/60 Sephacryl S-300 HR column (Cytiva, USA) was used, with the elution being performed with 50 mM sodium phosphate buffer, 150 mM NaCl, pH 7.2, at a flow rate of 0.5 mL min−1. The collected fraction of the purified monomer was then concentrated to 5 mg mL−1, using the amicon ultra-15 centrifugal filters (Merck, New Jersey, USA).
Protein aggregation was then induced to the purified mAb solution, in 1.5 mL eppendorfs, using different types of aggregation factors such as time, temperature, low pH shift or freeze–thawing (F/T). Time aggregates were generated by storing the mAb purified sample at 4°C for, at least, 4 weeks. Temperature aggregates were induced by incubating the mAb purified sample at 75°C for 10 min, using a thermomixer (Eppendorf, Hamburg, Germany) operated at 600 rpm. Low pH shift aggregates were produced by dialyzing the mAb purified sample with 50 mM sodium citrate buffer, 500 mM NaCl, pH 3, using the amicon ultra-15 centrifugal filters. F/T aggregates were generated by incubating the purified mAb sample at −80°C for 1 h followed by thawing at 25°C. The freeze–thaw cycle was repeated five times.
2.2 Characterization of the stressed formulations
After the different types of aggregates were generated, an initial characterization of each sample was performed using the following analytical techniques:
2.2.1 SEC-UPLC
The mAb concentration and the percentage of aggregation of each stressed sample is determined by analytical SEC in an UltiMate 3000 UHPLC System (Thermo Fisher Scientific, Massachusetts, USA). Five microliters of sample was injected in an ACQUITY UPLC Protein BEH SEC 200 Å column (Waters, Massachusetts, USA) and run with the 100 mM sodium phosphate buffer, pH 6.8, for 10 min. The flow rate used was 0.3 mL min−1 and the protein was detected at 280 nm.
2.2.2 Hydrophobic interaction chromatography (HIC)
The hydrophobicity of each stressed formulation was assessed by HIC, according to Goyon et al.16 A HiTrap™ Butyl FF column (CV of 1 mL), purchased from Cytiva (New Jersey, USA), was employed. An adsorption buffer of 3.5 M ammonium sulfate and 0.1 M phosphate buffer, pH 7, and an elution buffer of 0.1 M phosphate buffer, pH 7, were used. A gradient was performed from 25% to 100% of the elution buffer in 20 CV at a flow rate of 1 mL min−1.
2.2.3 Dynamic light scattering (DLS)
The presence of larger aggregates was determined by DLS, performed in Zetasizer APS with the protein size standard operating procedure (SOP) of the Zetasizer Software (Version 8.02, Malvern Panalytical, United Kingdom). One hundred microliters of each of the stressed formulations were measured in a 96-well plate at the fixed angle of 90°, at a laser wavelength of 830 nm and a temperature of 25°C. The samples were measured in triplicates and each sample was measured three times by the instrument.
2.3 HT screening of FDs
The HT screening of the selected FDs was performed in a Tecan EVO Freedom 200 robotic station (Tecan, Switzerland), equipped with a plate reader (InfiniTe Pro 200), a robotic manipulator (RoMa) arm (to move microplates to the different positions) and one liquid handing arm (LiHa). Corning 96-well NBS™ microplate (Merck, New Jersey, USA), made of white polystyrene, were used for the measurement of the fluorescence emission spectra. From the FDs stock solutions previously prepared, several diluted FD solutions were prepared with MilliQ water, with the concentration range tested present in Table 2. Then, 100 μL of each of the generated aggregates (maintaining the concentration of 5 mg mL−1) were pipetted into a well by the liquid handling robot, followed by the addition of 100 μL of the FD. Hence, a one-to-one ratio of sample to FD was used, which would be later applied in the micromixer measurements. After, the microplate is transported to the plate reader, where it is mixed at 600 rpm for 2 min and the fluorescence spectrum of each sample recorded according to the wavelengths described in Table 2. Extra blank measurements were performed where the mAb aggregate samples were replaced by buffer only. Then, the fluorescence signal recorded was subtracted from each mAb induced aggregation sample measurement to remove any buffer interference on the fluorescence signal.
| Fluorescent dye | Excitation wavelength (nm) | Emission wavelengths (nm) | Concentration range tested | Optimal concentration |
|---|---|---|---|---|
| ThT | 415 | 465–600 | 1–5 mM | 1 mM |
| CCVJ | 435 | 465–650 | 0.5–50 μM | 1 μM |
| Bis-ANS | 380 | 450–600 | 0.5–5 μM | 0.5 μM |
| Nile Red | 550 | 600–750 | 25–100 μM | 75 μM |
2.4 Aggregate detection with the micromixer
2.4.1 Structure fabrication
The zigzag microfluidic device (100 μm high × 100 μm wide × 17.2 mm long) presents two inlets and one outlet, each 100 μm wide (Figure 1).13 The dimensions of this micromixer are also described in Table 1. The designed mold was ordered from INESC Microsystems and Nanotechnologies (Lisbon, Portugal) and the structures were fabricated according to Gokaltun et al.17 to reduce the inherent hydrophobicity of PDMS. Dimethylsiloxane-(60%–70% ethylene oxide) block copolymer, comprised of poly(ethylene glycol) (PEG) and PDMS segments (PDMS-PEG), were blended with PDMS during device manufacturing, using a 10:1:0.0025 mixture of PDMS, curing agent and PDMS-PEG. After being degassed, the mixture is poured onto the mold and baked at 70°C overnight. After the PDMS is cured, the chip is removed from the mold and the inlets and outlet are punched. Finally, the PDMS chip is bonded to a glass substrate and sealed with a 20:1 mixture of PDMS to curing agent.
2.4.2 UV–Vis measurement
A syringe pump KD Scientific 200 (KD Scientific Inc, Massachusetts, USA) was used to pump both mAb sample and FD into the micromixer structure, with a flow rate of 1 μL min−1. The FD concentrations used were the optimal concentrations determined in the HT screening, present in Table 2. Before starting the measurement, the UV signal is first auto-zero resorting to a solution composed of the FD with the sample buffer, with a ratio of one-to-one. The autozero is performed to eliminate any interference from the dye's intrinsic fluorescence. After the two fluids were mixed in the microstructure, the fluid is sent to an inline UV–Vis detector (SPD-20AV, Shimadzu, Kyoto, Japan) which contains a microflow cell (0.2 μL). Two wavelengths were selected to perform the measurement for each FD, taking into consideration the results previously obtain in the HT screening: CCVJ, with excitation wavelength (λexc) of 435 nm and the emission wavelength (λem) of 520 nm; ThT, with λexc of 415 nm and λem of 520 nm; Bis-ANS, with λexc of 380 nm and λem of 520 nm; and Nile Red, with λexc of 550 nm and λem of 650 nm. After the connection of the micromixer to the UV–Vis detector, the aggregation measurement starts and the UV signal is recorded until is stable for at least 10 min. Then, after the signal stabilization, the micromixer is disconnected from the UV–Vis detector and the UV signal is once more autozero with the FD and sample buffer mixture. This procedure was repeated for all generated aggregate samples with each of the FDs selected in this study.
2.4.3 AEX validation
An ÄKTA™ Avant unit was used to perform an anion exchange chromatography (AEX) for the removal of aggregates, resorting to a 1 mL Capto™ adhere column (Cytiva, Massachusetts, USA). This system was equipped with: three pumps (pumps A, B and sample pump) with inlet valves in each to be able to select different buffers, a column valve, an outlet valve, three versatile valves, two UV monitors and a 10 mL Superloop™ (Cytiva, Massachusetts, USA). The sample pump was employed to inject the FD into the micromixer structure, using a flow rate of 3 μL min−1 (0.003 mL min−1). The versatile valves were used to incorporate the superloop and the micromixer in the system. The superloop will collect the FT and eluate from the AEX column, and then send it to the micromixer channel for aggregate detection. Thus, the reduction of the pump flow rate to 3 μL min−1 necessary for aggregate detection in the micromixer can be achieved. Additionally, two UV monitors were employed: UV1, placed after the micromixer, to detect the increase of signal due to the presence of HMW species; and UV2, placed after the column valve, to monitor the chromatographic run. The chromatography system was controlled with the research software Orbit, a control and data acquisition system developed at Lund University (Lund, Sweden). Orbit communicates with UNICORN™ and creates the necessary instructions to run a continuous process, which then sends the information to an ÄKTA™ unit. More information on the software Orbit is described elsewhere.18, 19
The buffers and flow rates used for the AEX chromatography run were based on GEHealthcare,20 for a sample concentration of 60 mg mL−1. The AEX operation was performed in flow through (FT) mode, with a mAb sample stressed by low pH induction, resulting in 6% aggregation. The selected FD was CCVJ ([CCVJ] = 1 μM, λexc = 435 nm, λem = 520 nm). Aggregate detection is performed during 60 min, after the collection of the FT and eluate from the AEX column in the superloop. Apart from the autozero of the UV signal, an extra phase was incorporated after aggregate detection where water is injected in the micromixer and in the connection tubes to clean and remove any remaining sample or FD.
3 RESULTS AND DISCUSSION
3.1 Aggregate induction and characterization
To create the necessary aggregation PAT tool, the designed micromixer should be able to detect the widest type and size range of aggregates which can arise during a biomanufacturing process. Thus, the first part of this work was to generate a broad sample variety, with aggregates possessing different physical and chemical characteristics, resorting to different induction factors. Using a mixture of mAb aggregate samples, the FDs and the micromixer will be later tested to assess the capability to detect all types of aggregates. The provided mAb already presented 4% of aggregation, becoming the first mAb sample to be tested, the hereby named storage aggregates. Then, to remove any remaining HMW species, SEC was performed and a purified mAb sample obtained, which unsurprisingly presented 0% of aggregation. Since mAb aggregate formation depends on the type of stress used,4 protein aggregation in the purified mAb sample was then induced using a variety of aggregation factors: time, temperature, low pH shift or F/T.
The level of aggregation was determined by SEC-UPLC (Table 3), ranging from 2.5% (time aggregates) to 10% aggregation (low pH aggregates). Additionally, SEC allowed to detect oligomers in the size range of 1–25 nm,2 with the presence of dimers and trimers across all aggregation samples. The presence of larger aggregates was then assessed by DLS, which can detect oligomers in the size range of 10 nm to 5 μm.2 The results obtained by this analytical technique are described in Table 3 and in Figure S1B. While the monomer mAb form presents a size of 12.5 nm, all the induced aggregates exhibit HMW species with larger sizes, especially the time and F/T aggregates (up to 1 μm). The storage aggregates similarly display larger dimensions, being mainly composed of trimers and HMW species up to 640 nm, as generally aggregates increase in size (and amount) in a time-dependent manner.2 Additionally, F/T as an aggregation induction factor, when performed with NaCl, forms larger particles (in the micron range) due to the decrease of colloidal stability.4
| Aggregation sample | Buffer | % Aggregation (SEC-UPLC) | Size (SEC-UPLC and DLS) | Hydrophobicity (HIC) |
|---|---|---|---|---|
| Purified mAb | 50 mM Na-PB Buffer, 150 mM NaCl, pH 7.2 | 0 | Monomer 165 kDa/12.5 nm |
− |
| Time aggregates | 50 mM Na-PB Buffer, 150 mM NaCl, pH 7.2 | 2.5 | Mainly dimers 375 kDa/320–1000 nm |
− |
| Storage aggregates | 25 mM NaOAc +5 mM NaCl, pH 4.5 | 4 | Mainly trimers 500 kDa/60–640 nm |
− |
| F/T aggregates | 50 mM Na-PB Buffer, 150 mM NaCl, pH 7.2 | 7 | Mainly dimers 375 kDa/20–40 & 640–1000 nm |
− |
| Temperature aggregates | 50 mM Na-PB Buffer, 150 mM NaCl, pH 7.2 | 6 | Trimers 500 kDa/40–80 nm |
+ |
| Low pH aggregates | 50 mM Na-Citrate +500 mM NaCl, pH 3 | 10 | Trimers 500 kDa/40–160 nm |
++ |
- Note: These samples were used in the HT screening of the FDs and in the UV–Vis detection with the micromixer. The type of aggregate induction, with the corresponding buffer used, the resulting percentage of aggregation (obtained by SEC-UPLC) and the characterization of each sample (achieved by DLS and HIC) is here described. More information on this characterization can be found in Supplementary Material (Figure S1). Na-Pb corresponds to sodium phosphate, NaCl to sodium chloride, NaOAc to sodium acetate and Na-Citrate to sodium citrate.
The mAb monomeric form and the resulting induced aggregates were also characterized according to their hydrophobicity by HIC.16 The results obtained are also presented in Table 3, with the corresponding chromatograms obtained in Figure S1A. In HIC, the retention of the molecule in the column is solely due to interactions between the surface amino acids and the stationary phase. Hence, different retention times and profiles are due to conformational changes, when compared to the monomer form. Temperature and low pH induced aggregates have a later retention time and profile, with the conformational changes suffered making them more hydrophobic when compared to the remaining samples. This increase in hydrophobicity happens when the antibody is exposed to strong destabilizing conditions, such as low pH (pH 3.0) and high temperatures (75°C): the level of unfolding exposes a larger number of hydrophobic patches.21 Nevertheless, a wide variety of different types of aggregates, ranging in terms of the level of aggregation, size and hydrophobicity, were generated. Since the type of stress that the mAb solution suffers greatly influences its physical and chemical properties,4 and in an integrated continuous process a variety of aggregation inducing factors are employed, it was crucial to have an extensive sample set. Since the objective is to create a PAT tool which covers the entire size range or type of aggregates, it is imperative that the micromixer (with the selected FD to be employed) can detect all mAb induced aggregation samples.
3.2 HT screening of FDs
Several commercially available FDs have been used to detect mAb aggregation.5, 8, 11 However, the minimal concentration required to produce a measurable signal for each FD when applied in the micromixer was still unknown. Hence, a HT screening of each FD was performed to determine the FD concentration to be employed in the micromixer. Additionally, possible constrains regarding the limit of detection of each FD can be assessed. A broad literature review on the use of these FDs was already performed in São Pedro et al.,12 which includes the concentration and wavelengths used in each reported publication. Based on this information, the concentration range tested is described in Table 2 as well as the excitation and emission wavelengths used.
The optimal concentration for each FD was reached (Table 2), with the fluorescence spectra recorded for the best condition represented in Figure 2. As expected, all FDs had strong fluorescence signals for the majority of the mAb induced aggregation samples, with most being able to distinguish as little as 2.5% of aggregation. Moreover, the purified mAb sample displayed almost no fluorescence intensity across all four FDs. Nevertheless, Bis-ANS and Nile Red, both hydrophobic sensitive FDs, do not produce a major increase in fluorescence intensity for the F/T aggregates, when compared to the mAb purified sample. The F/T aggregates do not contain any exposed hydrophobic regions in the unfolded aggregated structure which would produce a fluorescence intensity increase by these two FDs. Previous studies indicate that the mAb native structure is retained to a high degree after F/T, with the sample mainly be composed of native-like IgG molecules.22 The HIC characterization confirms this hypothesis since the hydrophobicity of the F/T aggregates did not increase compared to the mAb monomer form, showing the same retention time and profile. Additionally, Nile Red also does not produce a fluorescence signal for the time aggregate detection, the sample with the lowest level of aggregation, which indicates the dye's limit of detection. Therefore, Nile Red will only be suitable to be applied in mAb samples, which present more than 2.5% of aggregation.

Furthermore, for all FDs employed, it is possible to observe that the fluorescence signal measured does not directly correlate with the quantity of mAb aggregates in the sample. For example, as seen in Figure 2a, the storage aggregates display a higher fluorescence signal than the remaining mAb samples, except the low pH aggregates. However, the storage aggregates only present 4% of aggregation. The fluorescence intensity not only depends on the amount of aggregates, but also on the properties of such aggregates.15 Ultimately, a straightforward quantification of aggregation based on the FD signal is, up to the moment, not possible. The aggregate measurement provided by the FDs and, subsequently, the micromixer, will only be qualitative, not quantitative.
3.3 Application of micromixer
3.3.1 UV–Vis sensor
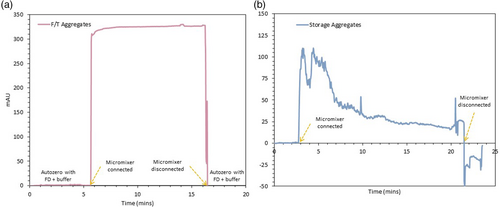
With the FD concentration defined, the next step was to validate the developed micromixer for mAb aggregation detection resorting to a standard UV–Vis detector. Each FD, with the mAb aggregation sample to be tested, were simultaneously pumped in the microfluidic structure where both streams mixed under 30 s. A flow rate of 1 μL min−1 was employed, which provides a mixing efficiency of around 90%.13 The resulting fluid is then sent to an inline UV–Vis detector where, if an increase of the signal is observed, aggregation is detected. The emission wavelength of each FD was selected according to the HT screening performed beforehand, choosing a wavelength close to the peak of the fluorescence intensity signal. Before starting the measurement, the UV signal is auto-zero with a solution composed of FD and the sample buffer (ratio one-to-one) to eliminate any possible interference from the FD intrinsic fluorescence. As observed in Figure 3a, after the micromixer is connected to the UV–Vis detector, the signal is allowed to stabilize for, at least, 10 min before finishing the measurement by disconnecting the micromixer and auto-zero again with the same FD and sample buffer solution. Similar procedure was repeated for all four FDs and all mAb aggregate samples, with the results obtained presented in Figure 4. However, not all FDs were able to be successfully employed in the UV–Vis detector. Nile Red, as observed in Figure 3b for the detection of storage aggregates, does not produce a stable signal over the imposed 10 min time range. The signal decreases during the measurement due to the aggregation of the FD. Nile Red self-associates in dimers in the micromixer and subsequently, in the tubes connecting to the UV–Vis detector, due to the dye's poor solubility in water.23 These aggregates interfere with the aggregate measurement and, since the majority of the buffers used during the mAb purification chain are composed by water, Nile Red cannot be applied as a FD to detect aggregation within a continuous integrated process. Therefore, Nile Red was discarded as a FD to be further used in this miniaturized PAT tool.
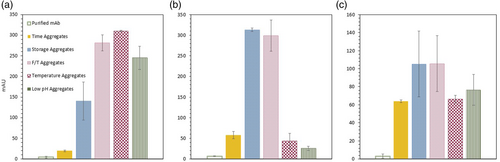
Nevertheless, all the remaining FDs, ThT, CCVJ and Bis-ANS, were able to successfully provide a stable measurement and detect aggregation (Figure 4). The purified mAb sample, with 0% aggregation, yields no measurable UV signal across all three FDs. Moreover, all the aggregate samples displayed great increases in the UV signal when the aggregation measurement started. Although ThT could detect all the different mAb induced aggregation samples, the UV signal from the temperature and low pH aggregates is relatively less than the remaining (storage and F/T aggregates). ThT binds to intermolecular β-sheets formed in high-order aggregates, being an indicator of amyloid structure.24, 25 Hence, the lower ThT signal indicates that the temperature and low pH aggregation mechanism is not accompanied by β-sheet formation, but by the exposure of the hydrophobic patches. The HIC results already showed the increase in the hydrophobicity of these two samples when compared to the mAb monomer form (Table 3). Therefore, since not all mAb aggregation mechanisms will lead to a β-sheet formation, ThT is no longer considered a suitable FD to be applied and will not be further explored. Nonetheless, Bis-ANS and CCVJ exhibit an ample increase in the UV signal and are suitable to be used in the micromixer. Once again, the UV signal obtained does not directly correlate with the amount of aggregates in each sample: for example, for Bis-ANS, the time aggregates produce a similar UV signal than for the temperature aggregates. Thus, FD detection of aggregation depends more on the proprieties of the aggregates than the amount, providing a merely qualitative measurement. The limit of detection (LoD) of the developed PAT tool is intrinsically connected to the LoD of the FD. Therefore, to properly use the developed PAT tool, further investigation into the LoD of the FD is needed. Additionally, in the past years, there has been the development of novel FDs, like Proteostat, which might be better suitable to detect other types of aggregates9 and might be an alternative option to be applied in the micromixer. Nevertheless, with CCVJ and Bis-ANS being able to produce measurable UV signals, it was demonstrated the potential of using the two FDs to successfully detect HMW species.
Finally, one of the design constrains when developing this fluorescent dye-based microfluidic sensor was that the micromixer would not alter the amount of aggregates.13 To confirm that indeed the micromixer was not affecting the sample's aggregation levels, the mAb aggregate samples were collected after the UV–Vis detection and analyzed by SEC-UPLC. No increase in the level of aggregation was observed for any analyzed sample (data not shown). Therefore, the developed microfluidic sensor fulfills all the design constrains of a PAT tool: a real-time measurement of mAb aggregation in a continuous process can be achieved, with the micromixer providing 90% of mixing efficiency within 30 s.13
3.3.2 AEX validation
The final evaluation of the fluorescent dye-based micromixer was to employ it in a chromatographic operation for aggregate detection. AEX chromatography was performed in a flow-through (FT) mode for the removal of aggregates, which has been previously optimized.20 The micromixer was then implemented in a standard ÄKTA™ Avant unit, with the addition of an extra UV sensor, one 10 mL superloop and three versatile valves (Figure 5a,b). The extra UV sensor (UV2) was used to monitor the chromatographic run whereas the UV already present in the ÄKTA™ unit (UV1) was placed after the micromixer to detect the aggregation signal provided by the FD. The superloop was added, after the chromatography column, to collect the FT and the eluate (Figure 5a). The pumps already existing in the system, pump A and B, were not only used for the chromatographic run but also to pump the FT/eluate collected in the superloop directly to the micromixer (Figure 5b). Therefore, to allow the reduction of flow rate for aggregate detection in the micromixer, the incorporation of the superloop was crucial. Due to pump limitations for lower flows, a flow rate of 3 μL min−1 was applied, which is still able to provide a high mixing efficiency (around 85%). Additionally, the sample pump (SampP) injects the FD directly into the micromixer, with a similar flow rate.
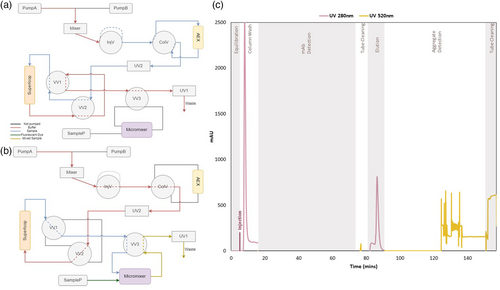
From the results obtained in the UV–Vis detector, CCVJ, a molecular rotor, showed a more significant UV signal increase, around 300 mAU, being the preferred FD to be used in this validation. A mAb sample, with a concentration of 60 mg mL−1, was stressed by low pH induction, obtaining a 6% level of aggregation (Table 4). This aggregation sample was then injected onto an AEX column, with the results obtained found in Figure 5c. First, after the column equilibration and sample injection, the FT is collected in the superloop. Then, a FT sample is sent to the micromixer, where when mixed with the FD, no UV signal was detected. Hence, the FT sample exhibits no aggregation, with AEX column being able to bind all the aggregates. The level of aggregation was later confirmed by SEC-UPLC (Table 4), which merely identified 0.4% of aggregates. Later, after cleaning the micromixer and the attached tubes with water and disposing the remaining sample in the superloop, the AEX elution was performed and collected. The detection procedure is repeated once more, and for the eluate, the UV signal increased. Even though theoretically the pump system can handle flow rates starting at 1 μL min−1, the ÄKTA™ system still has some limitations when using lower flow rate. The presence of air bubbles is visible on the UV signal, especially in the first minutes after the signal increases. Nevertheless, the signal was allowed to stabilize and aggregation was still detected using the micromixer sensor. The detection time was initially defined to be 60 min which needs to be considerably reduced to provide a real-time analysis. Since the micromixer can provide an efficient mixing under 30 s,13 modifications to the external ÄKTA™ setup have to be performed to decrease of the overall measurement time. For example, by reducing the connection tubes which connect the versatile valve to the micromixer, this decrease of measuring time can be achieved. The aggregate detection was again confirmed by SEC-UPLC, with the eluate collected presenting 5% of aggregation (Table 4). Thus, even if a long measuring time was defined, the proposed micromixer was still able to successfully detect aggregation.
| Aggregation (%) | Concentration (mg mL−1) | |
|---|---|---|
| Initial sample | 6 | 60.0 |
| FT sample | 0.4 | 2.0 |
| Eluate sample | 5 | 0.5 |
Recently, with the development of the PAT framework, several PAT tools have been successfully implemented to detect aggregation in the required time frame for decision making and control.26, 27 For example, Patel et al.28 has used a multi-angle light scattering (MALS) system coupled to ÄKTA™ unit to real-time quantify the formation of aggregates. However, extra equipment such as a MALS system is not as readily available as a standard UV–Vis detector to detect aggregation. If the detection time can be significantly decreased, the increase in the UV signal can be used as a cut-off point to stop collecting the mAb product. When developing this PAT sensor, several design constraints were imposed including: the overall cost of the technique had to be minimal and the microfluidic chip simple to operate.12 By using a zigzag micromixer and a simple extra UV monitor connected to a ÄKTA™ unit, these design constraints are met since no external equipment and setup is required, which would increase the cost and complexity of the developed PAT tool. Although FDs have inherent limitations regarding the quantification of the HMW species, a simple zigzag micromixer was firstly designed13 and hereby applied and tested. Thus, this work demonstrates that the miniaturization of the analytical technique discussed by São Pedro et al.12 is a powerful solution to speed up the CQAs measurement in a continuous process.
4 CONCLUDING REMARKS
A PAT fluorescent dye-based microfluidic sensor was developed and hereby introduced, being able to detect all different types of aggregates tested. From a SEC purified mAb sample, with 0% of aggregation, several induction factors were used to create a large variety of mAb aggregates, which presented different physical and chemical properties. A HT screening was then performed to assess the required concentration, emission wavelength and limit of detection of each FD later to be applied in the micromixer. This microfluidic chip was then connected to an UV–Vis detector and tested to detect mAb aggregation with all the stressed samples. Even though Nile Red and ThT were not suitable to be applied due to intrinsic limitations of the dye, Bis-ANS and CCVJ provide a measurable signal when aggregates were present in the analyzed sample. However, a measurement resorting to FDs will merely be qualitative, not yet being possible to quantify aggregation based on its signal. The FD signal is more dependent on the type of aggregate than its amount, making this developed PAT tool able to solely detect protein aggregation. Ultimately, the micromixer was validated in an AEX chromatographic run for the removal of mAb aggregates. An increase of the UV signal was observed on the eluate sample, which presented 5% of aggregation, whereas the FT sample, with 0.4% of aggregation, was not (Figure 5c and Table 4). Even though further investigation into the LoD of each FD should be performed, it was demonstrated that the micromixer can efficiently and robustly detect several type of aggregates and can be easily incorporated in a downstream unit operation.
Although the micromixer was able to successfully detect aggregation in a chromatography run, the measurement was still performed for 60 min. To create a real-time measurement, this detection time ought to be significantly reduced. By decreasing the connection tubes length in the ÄKTA™ system and/or introducing an extra phase in the Orbit software to fill these tubes with sample prior to the measurement, this time reduction should be achieved. Nevertheless, a fluorescent dye-based microfluidic sensor was demonstrated, being able to effectively detect a wide range of mAb aggregates. Furthermore, the micromixer was capable of handling the higher flow rates and pressure inherent to the ÄKTA™ system, demonstrating the potential of miniaturizing the analytical technique to accelerate CQAs measurement to the required time frame for process control.
AUTHOR CONTRIBUTIONS
Mariana Neves São Pedro: Investigation (lead); methodology (lead); validation (lead); writing – original draft (lead). Michel H. M. Eppink: Funding acquisition (equal); methodology (supporting); project administration (supporting); supervision (supporting); writing – review and editing (equal). Marcel Ottens: Funding acquisition (lead); methodology (supporting); project administration (lead); supervision (lead); writing – review and editing (equal).
ACKNOWLEDGMENTS
The authors wish to thank the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 812909 CODOBIO, within the Marie Skłodowska-Curie European Training Networks framework.
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1002/btpr.3355.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.